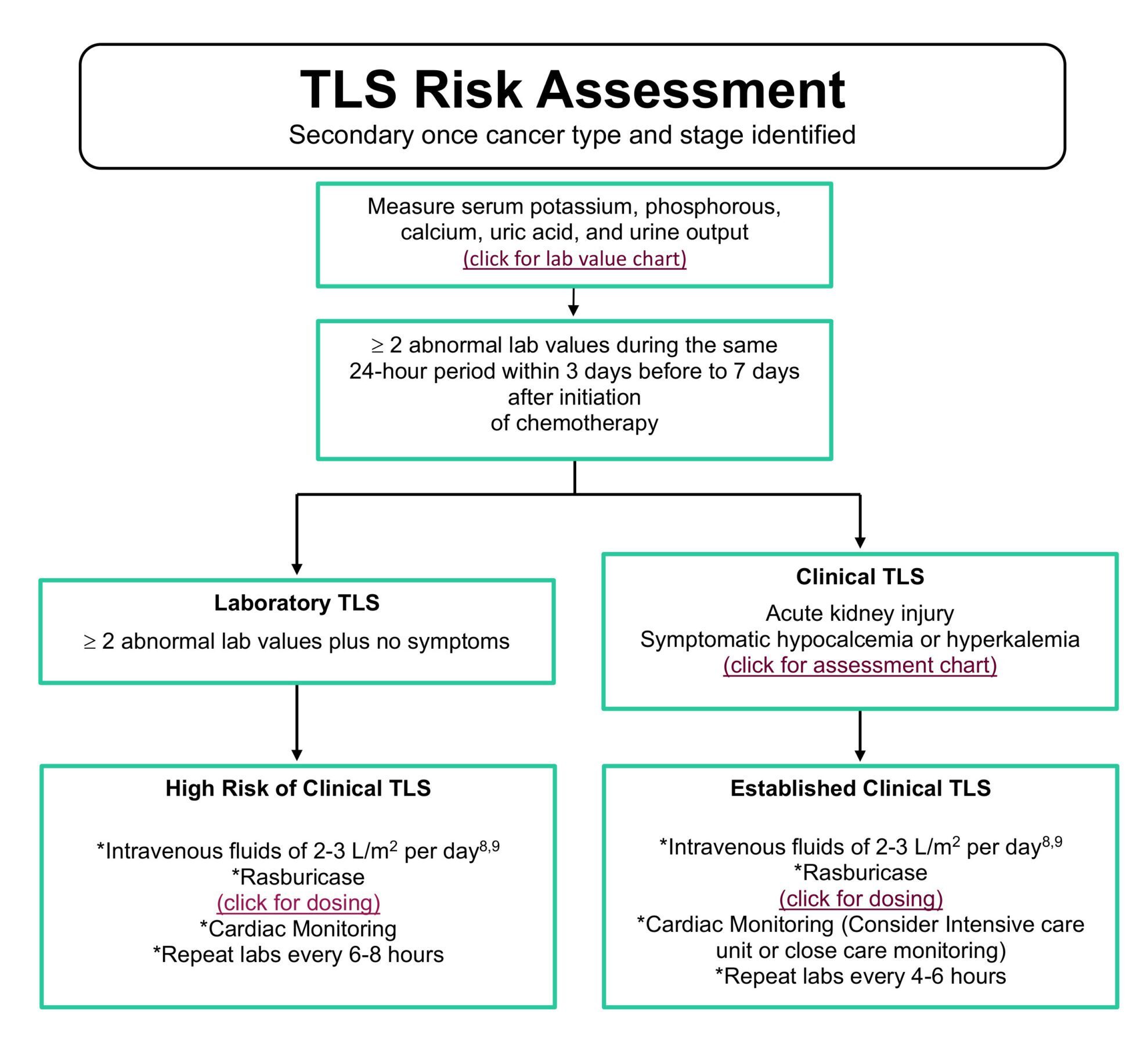
Tumor Lysis Syndrome (TLS) results from the rapid breakdown of cancer cells, eliciting metabolic imbalances in the bloodstream including hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3 Cancer patients receiving chemotherapy and/or radiation can also be at a higher risk for TLS depending on their cancer type, tumor burden, dehydration or impaired renal function.3 Performing a TLS risk assessment can proactively assist healthcare providers to identify patients who may require additional hydration and/or medication to mitigate or prevent life-threatening complications.1,3-4
Elitek ® (rasburicase), approved to treat hyperuricemia associated with malignancy in adult and pediatric patients, is an option for treatment, especially in cases of high risk for developing tumor lysis syndrome.5 Rasburicase, a recombinant urate-oxidase, works within 4 hours of administration to break down uric acid and prevent accumulation in the body in comparison to the standard of care treatments that target the formation of uric acid and elicit a slower onset of action.5,6 Standard dosing of Elitek® is determined by TLS risk assessment outcomes: typically patients exhibiting symptoms in intermediate and high risk categories.4-9 Single fixed dosing or one time weight-based rounded dosing is currently off-label but widely used and accepted as effective treatment. The single, reduced dosing is based on patient serum uric acid levels and has been shown to be a cost-effective alternative in lowering initial uric acid levels to reduce TLS risk.10-16
NCODA has created a tool to assist members on assessing the risk of TLS in adult and pediatric patients to determine if rasburicase treatment should be initiated. The user will be asked to answer a series of questions relating to patient oncolytic disease type, tumor burden and current underlying conditions to determine if rasburicase treatment is warranted.
TLS Risk Assessment - by Cancer Type and Stage
What cancer type are you treating?
- Acute Lymphocytic Leukemia (ALL)
- Acute Myeloid Leukemia (AML)
- Burkitt Lymphoma (BL)
- Chronic Lymphocytic Leukemia (CLL)
- Chronic Myeloid Leukemia (CML)
- Diffuse Large B-cell Lymphoma (DLBCL)
- Multiple Myeloma (MM)
| Risk Stratification by Disease State | |||
|---|---|---|---|
| Disease State | Low Risk | Intermediate Risk | High Risk |
| ALL | N/A | WBC < 100,000 and LDH < 2 x ULN (If YES to above click here) |
WBC ≥ 100,000 (adults) or ≥ 50,000 (peds) and/or LDH ≥ 2 x ULN (peds consider LDH > 500units/L) (If YES to above click here) |
| AML | WBC < 25,000 and LDH < 2 x ULN (If YES to above click here) |
WBC 25,000 to 100,000 or WBC < 25,000 and LDH ≥ 2 x ULN (If YES to above click here) |
WBC ≥ 100,000 (adults) or 50,000 (peds) and/or LDH ≥ 2 x ULN (peds consider LDH > 500units/L) (If YES to above click here) |
| BL | N/A | LDH < 2 x ULN (If YES to above click here) |
BL stage III/IV and/or LDH ≥ 2 x ULN (If YES to above click here) |
| CLL | WBC < 50,000 and Treated only with alkylating agents (If YES to above click here) |
ALC ≥ 25,000 and/or WBC ≥ 50,000 or Treatment with fludarabine, rituximab, or lenalidomide, or venetoclax and lymph node ≥ 5 cm (If YES to above click here) |
Treatment with venetoclax and lymph node ≥ 10 cm or Lymph node ≥ 5 cm and absolute lymphocyte count ≥ 25,000 and elevated baseline uric acid (If YES to above click here) |
| CML | Any stage | N/A | N/A |
| DLBCL | Intermediate grade DLBCL and LDH within normal limits (If YES to above click here) |
LDH > ULN or Bulky disease (If YES to above click here) |
Bulky disease and LDH ≥ 2 x ULN (If YES to above click here) |
| MM | Any stage | N/A | N/A |